Quantifying Heterogeneity in the Causal Impact of Abortion Restrictions
UC Santa Barbara
2025-04-23
Research Team

Elizabeth Stuart
Statistician, Hopkins

Avi Feller
Statistician, UC Berkeley

Alison Gemill
Demographer, Hopkins

Suzanne Bell
Demographer, Hopkins

David Arbour
Statistician, Adobe

Eli Ben-Michael
Statistician, Carnegie Mellon
Texas Senate Bill 8
Effectively bans abortion
Sept. 1, 2021
Roe v. Wade overturned
June 24, 2022
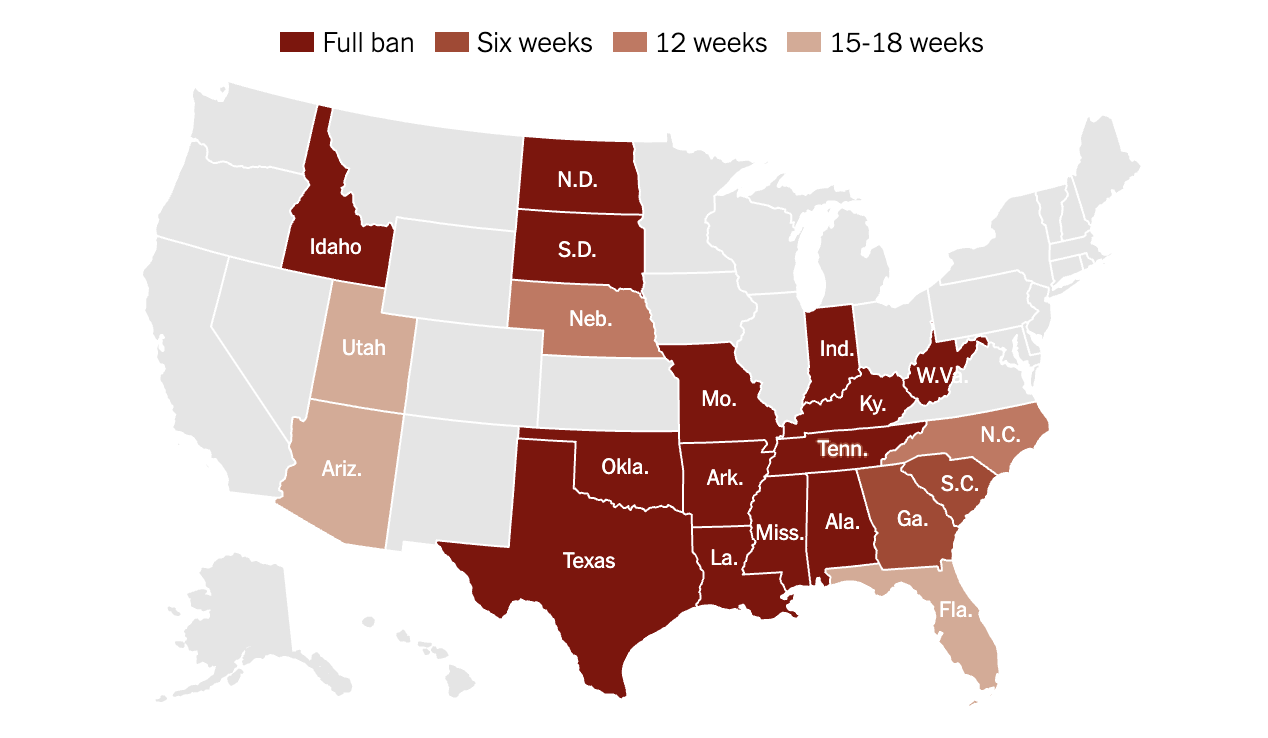
Abortion Bans Across the US
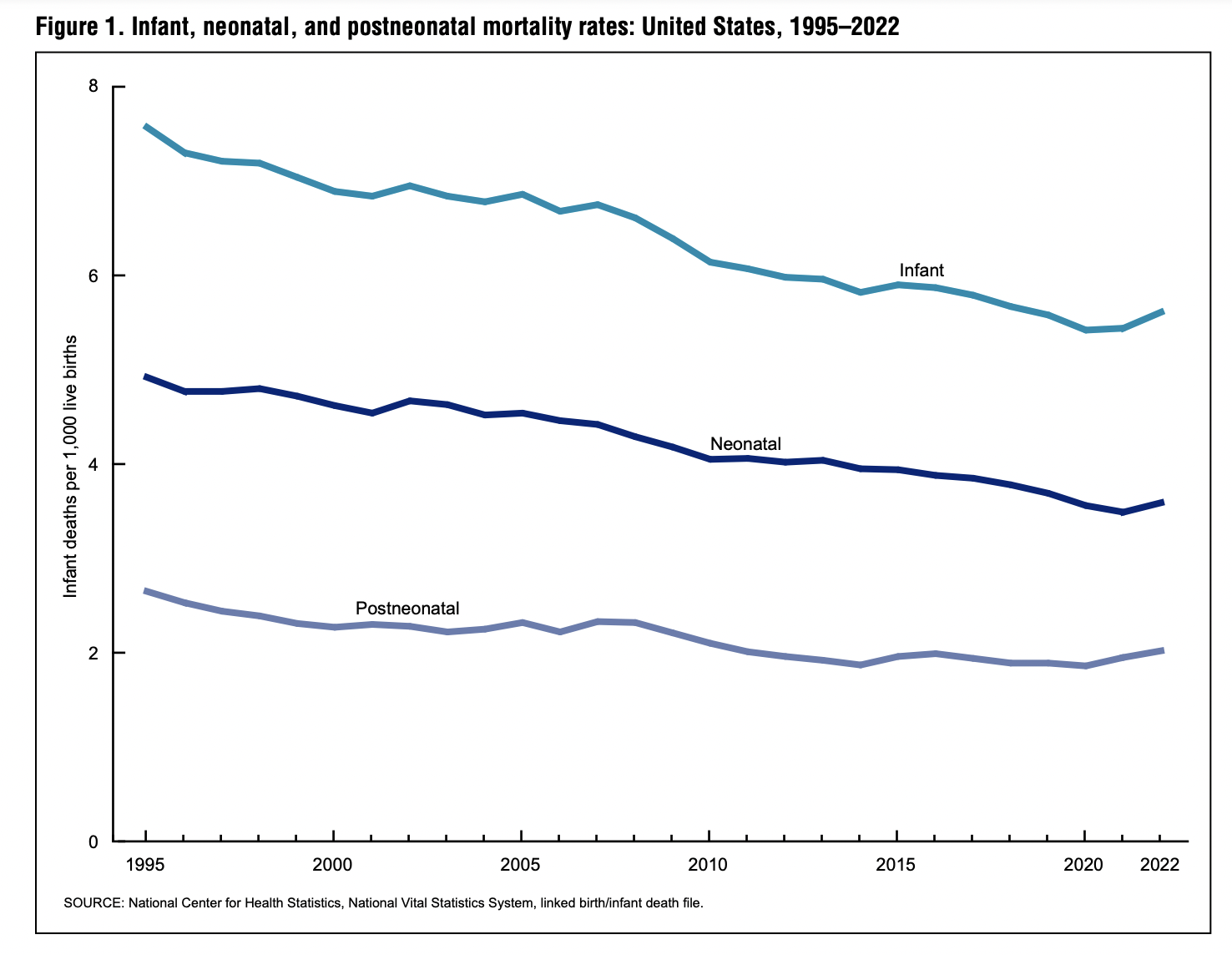
US Infant Mortality Rates
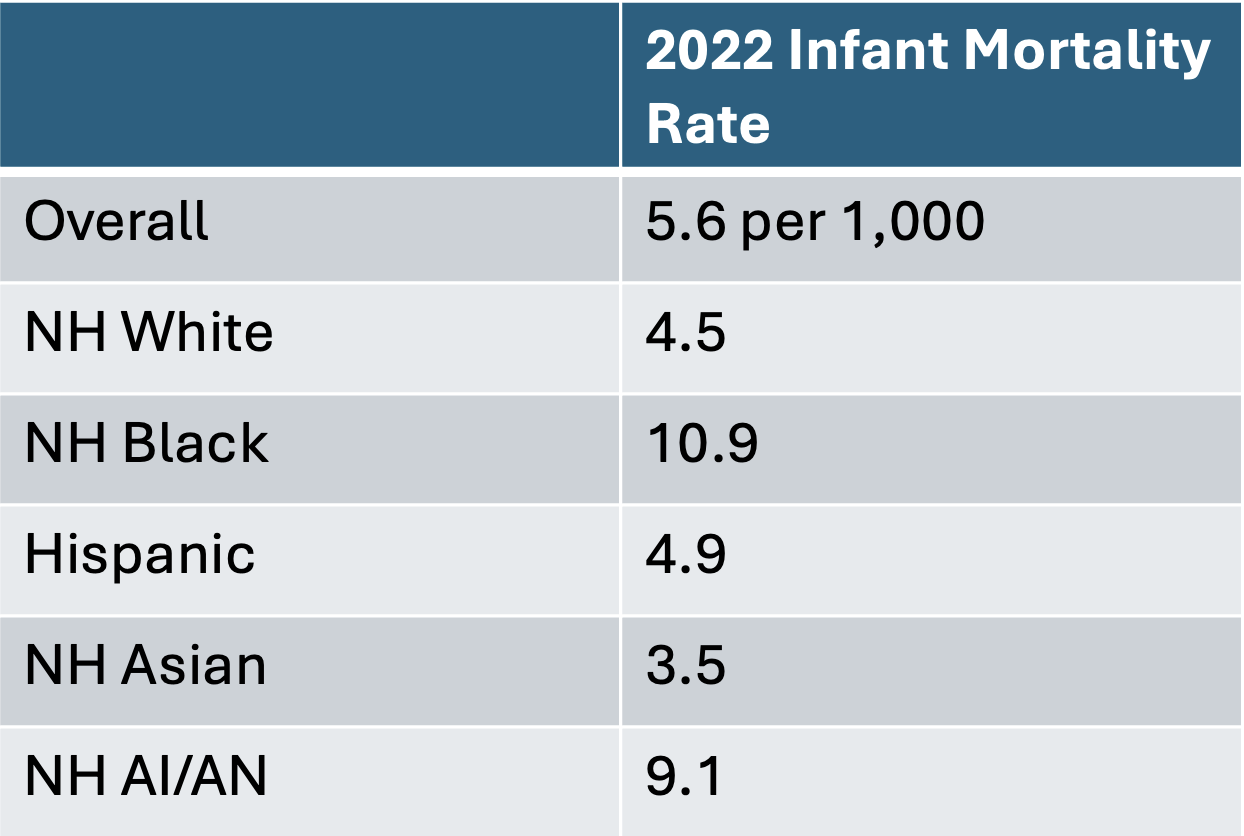
Early Evidence on Impacts
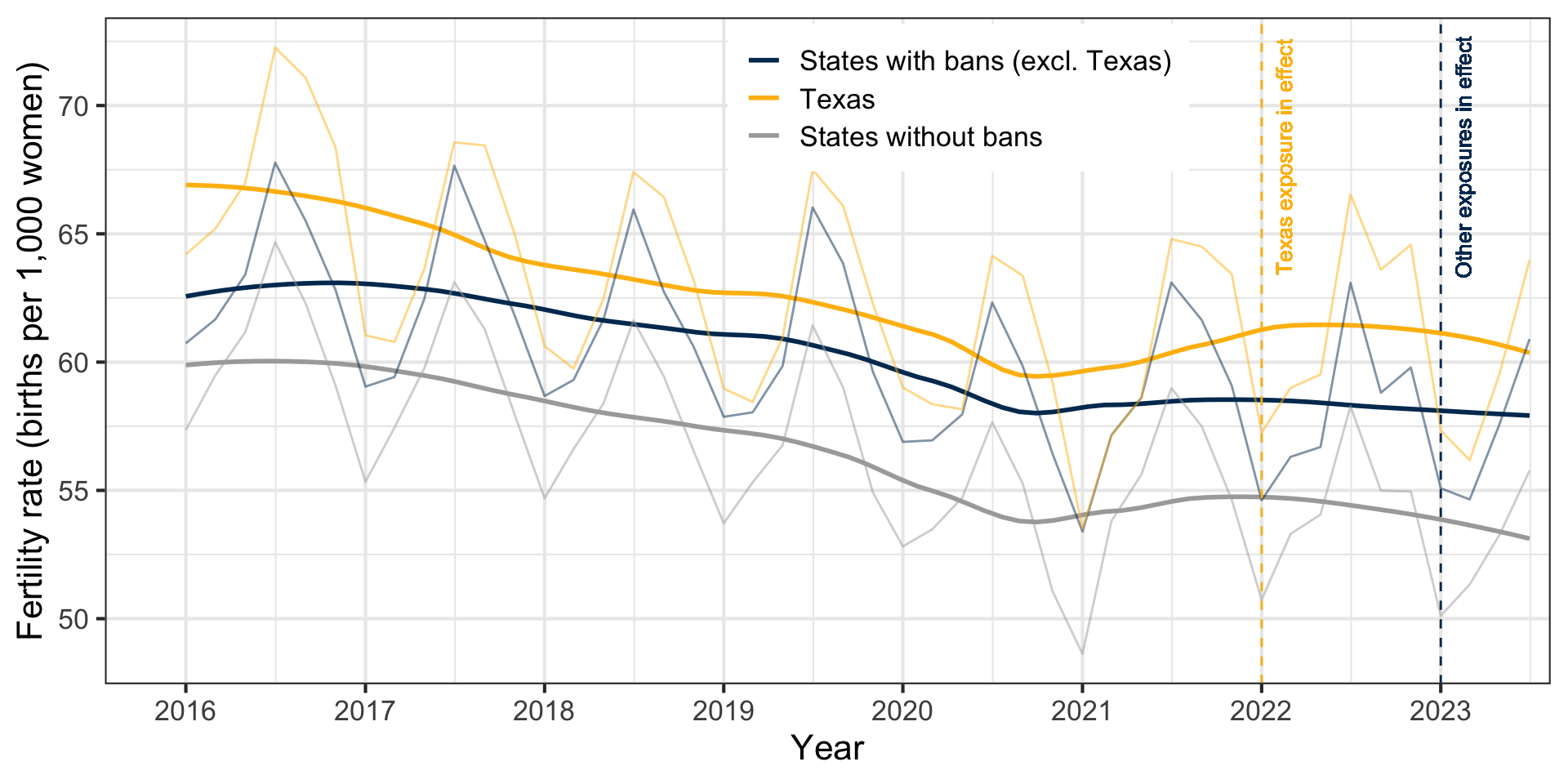
States with abortion bans experienced an average 2.3% increase in births in first half of 2023 (Dench, Pineda-Torres, and Myers 2024)
By race/ethnicity: greater impact among non-Hispanic Black and Hispanic individuals (Dench, Pineda-Torres, and Myers 2024; Caraher 2024) and greater impact among 20-24-year-olds (Dench, Pineda-Torres, and Myers 2024)
~13% increase in infant deaths; 8% increase in the infant mortality rate (Gemmill et al. 2024)
Study Objectives
To estimate sociodemographic variation in the impact of abortion bans on subnational birth rates in the US through the end of 2023
- By age, race/ethnicity, marital status, educational attainment, insurance type
- To estimate variation in the impact of abortion bans on subnational infant mortality in the US through the end of 2023
- By race/ethnicity, timing of death, cause of death
Fertility Trends
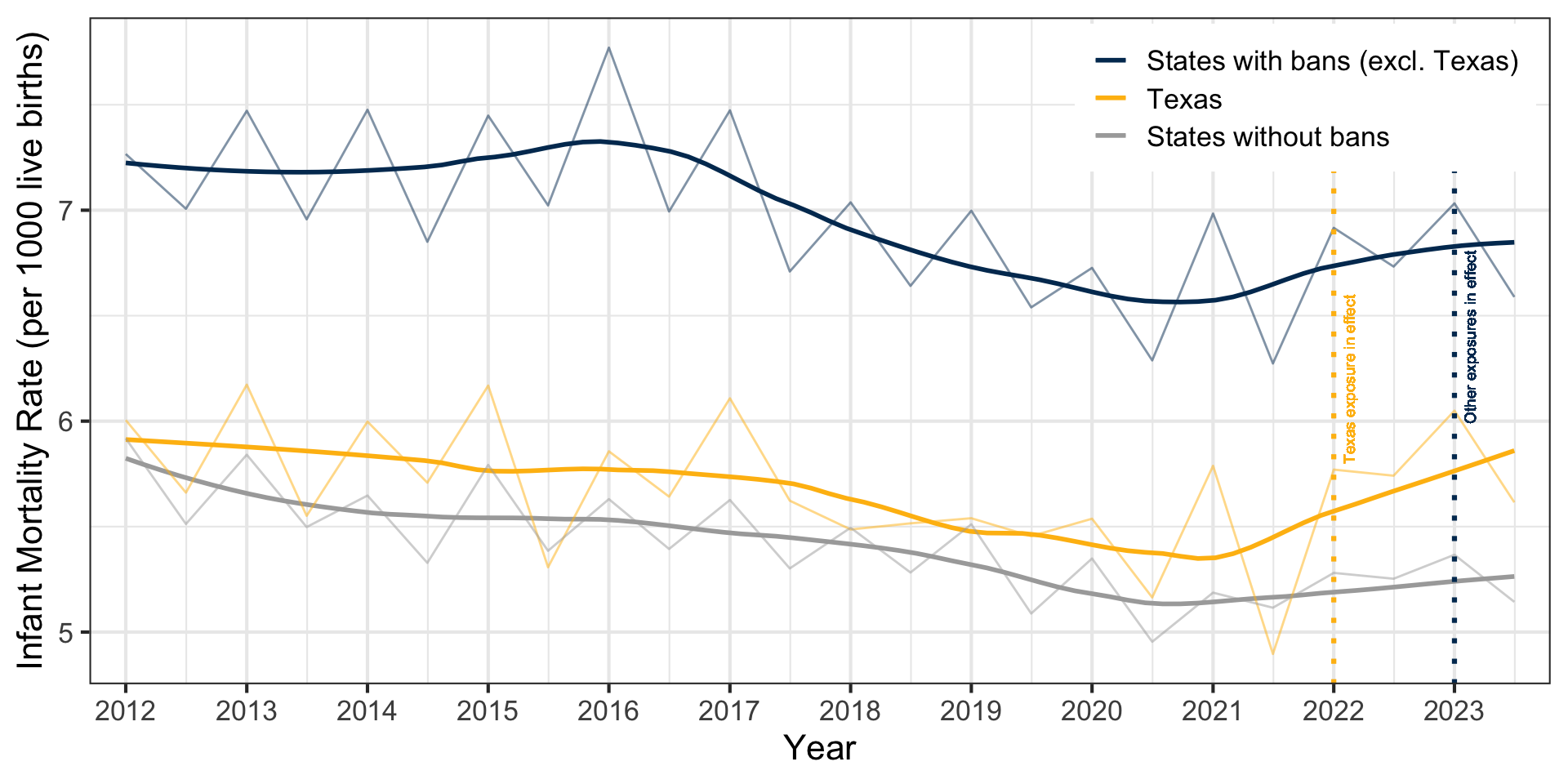
Infant Mortality Trends
Overall Analytic Approach
- Today: focus methods discussion on infant mortality data
- Models for the fertility data are very similar
- Bayesian panel data approach
- Poisson latent factor model
- Fertility: model bimonthly number of births with population offset
- Infant mortality: model biannual number of deaths with live birth offset
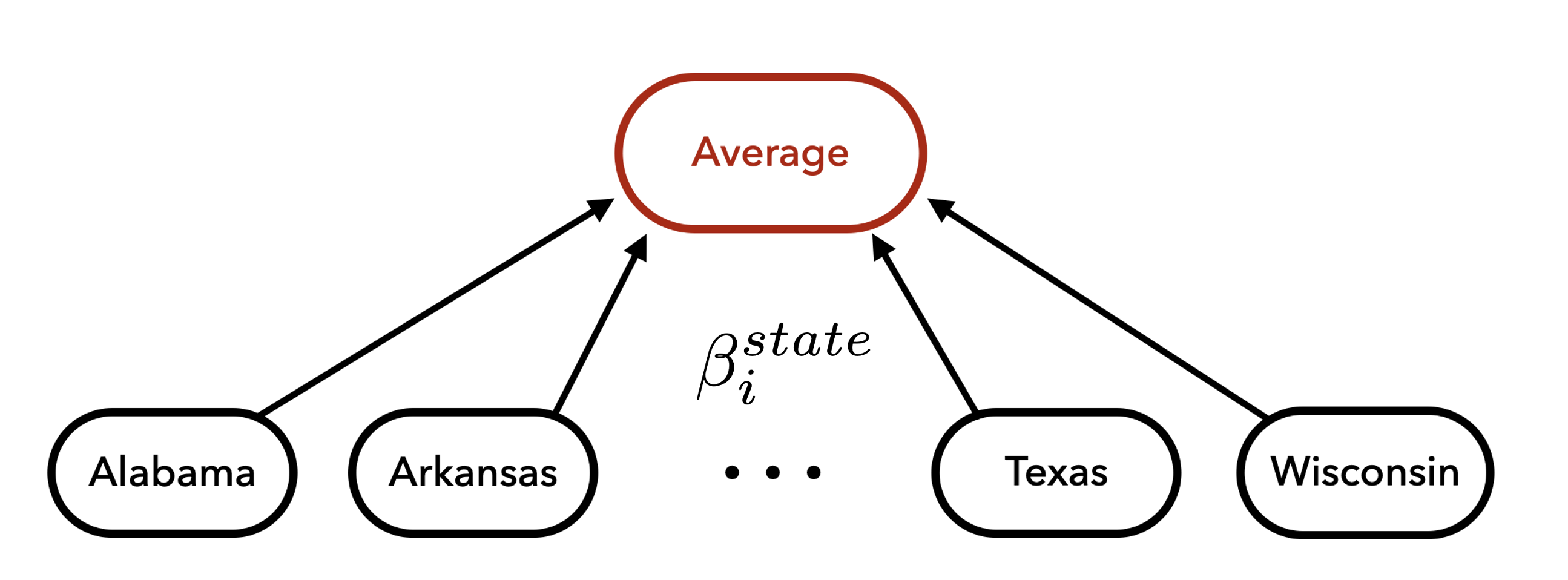
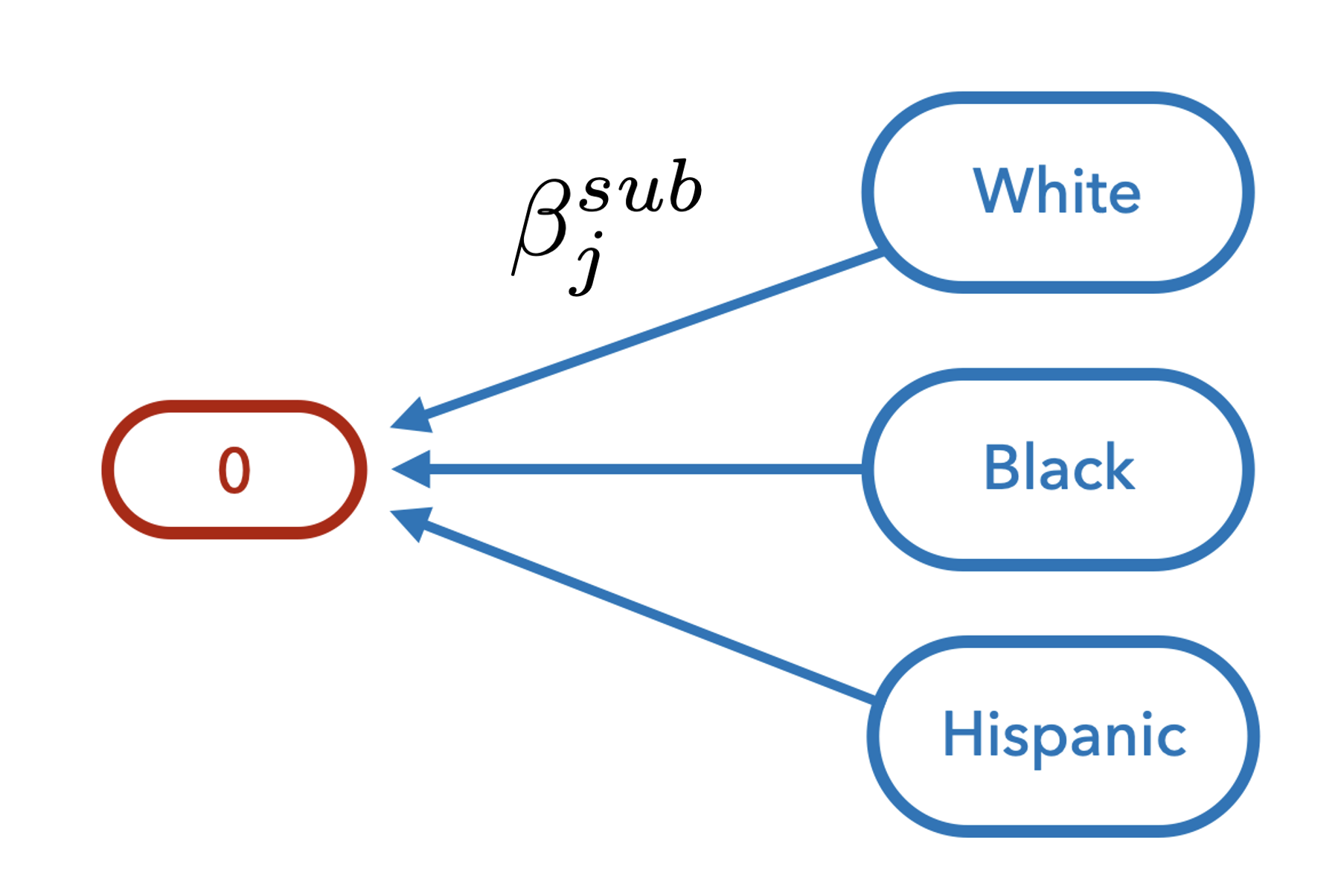
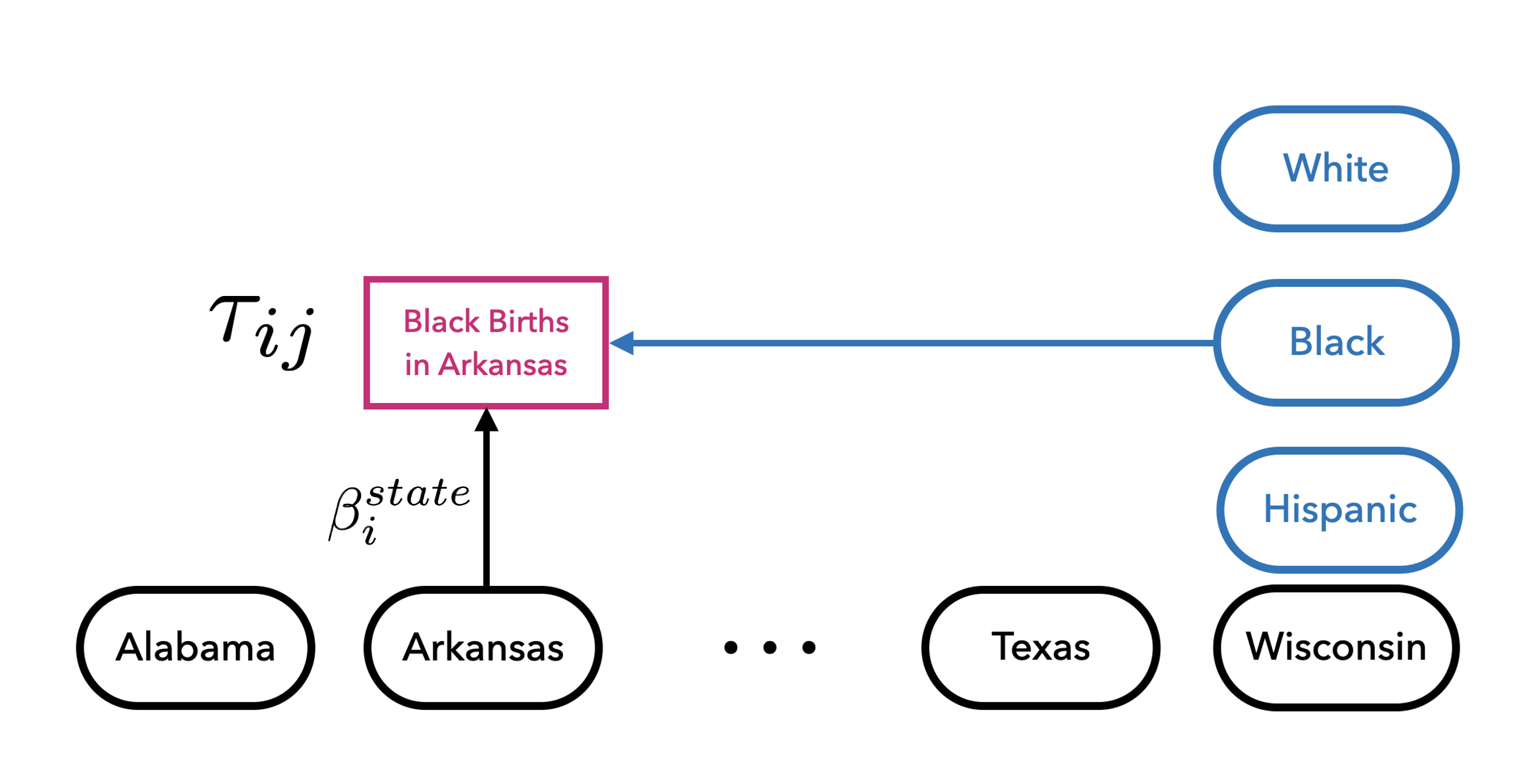
- Model state-by-subgroup-specific impacts separately by characteristic
- States without bans and pre-exposure outcomes in all states inform counterfactual
Infant Mortality Approach
- Outcome: infant mortality rate (deaths per 1,000 live births)
- Exposure: 6-week or complete abortion ban (14 states1), staggered adoption
- Pre-policy period: January 2012 through ~December 2022
- Treated period: ~January 2023 through December 2023
- Subgroups
- Race/ethnicity: non-Hispanic White, non-Hispanic Black, Hispanic, and Other
- Timing: neonatal (<28 days), non-neonatal
- Cause of death: congenital, non-congenital
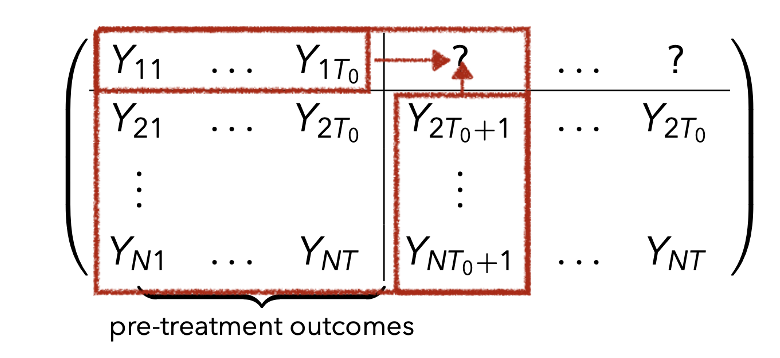
Panel Data
- Panel with
- Potential outcomes
- We observe for each unit the pair
Causal Inference for Panel Data
Assumptions:
Well-defined exposure: {any complete or 6-week abortion ban} vs {no ban}
No anticipation: no effect of abortion restrictions prior to exposure
No spillovers across states: outcomes only depend on own state’s policy

Causal Inference for Panel Data
Some common strategies:
- Interrupted Time Series (horizontal)
- Synthetic Control Methods and Factor Models (vertical)
- Differences in Differences(DID) and Two-Way-Fixed-Effects (TWFE)
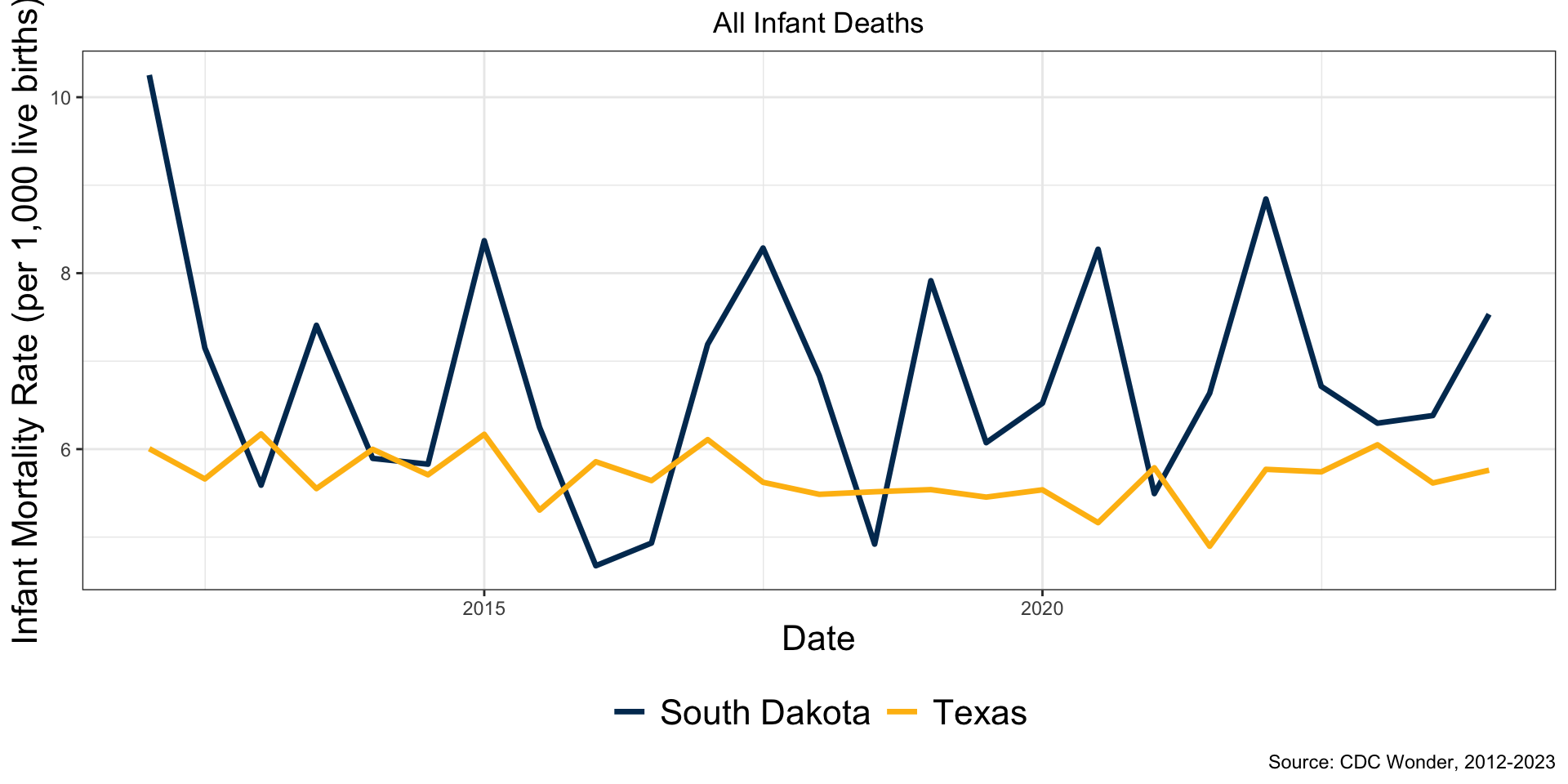
Challenges with Infant Death Data
- Infant death counts are small and discrete
- Missing data: CDC Wonder excludes counts between 1 and 9
- Implications for level of temporal aggregation
- States and subgroups vary in size and mortality rates
- Staggered Adoptions
- Bans were imposed at different times
Temporal Aggregation
- Missingness → CDC Wonder suppresses counts 1, …, 9 (but not 0!)
- e.g., annual → no missingness; daily → high missingness
- Later: imputation approach
- Noise → noise for (avg) annual counts ≪ (avg) monthly counts (see Sun, Ben-Michael, and Feller 2024)
- Further complicated by seasonality
- Fertility → 2 month intervals (e.g., Jan-Feb 2023)
- Mortality → 6 month intervals (e.g., Jan-June 2023)
Subgroup Inference
Summing infant deaths over subroups yields total infant deaths
Inferred total infant mortality rates by differ depending on which subgroups are considered
Better to estimate the total effect by estimating the subgroup effects and summing or modeling the total effect directly?
State Size and Sampling Variance
Subgroup Size and Variability
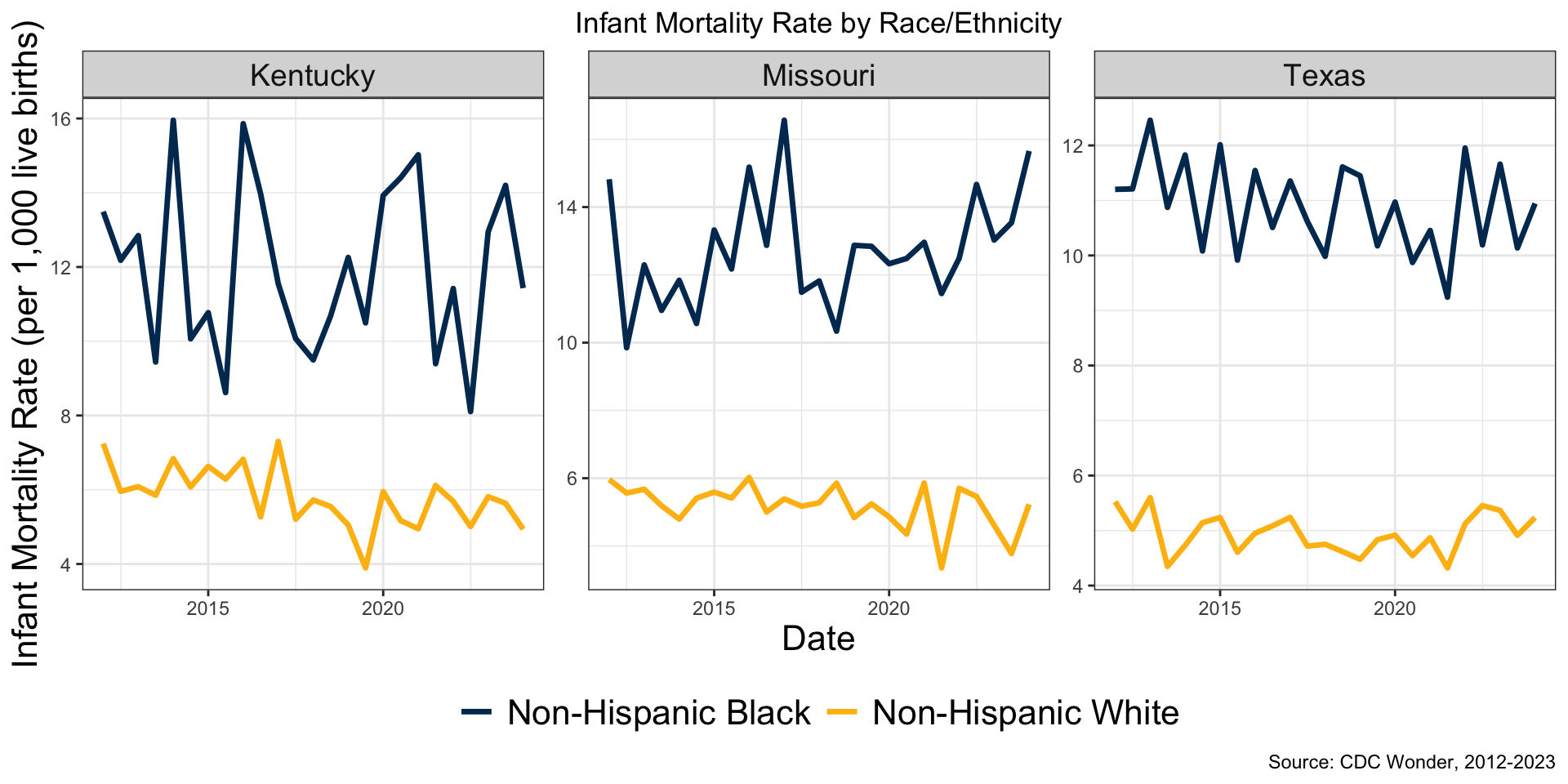
In these states population white ≈ 5-15x population black
Implications
Pre-treatment balance should depend on state and subgroup size
Avoid overfitting to noise when groups are small
The difference between realized and counterfactual infant deaths,
Suggests a need to regularize causal effect estimates
Want to encourage estimated infant mortality rates to be similar for the same state or same subgroup, while still allowing for the possibility of differences
A Probabilistic Bayesian Model
- Explicitly incorporate a missing data model
- Staggered adoption accounted for in the likelihood
- Count data modeled via Poisson with offset based on state/group size
- Hierarchical prior stabilize treatment effect estimates and partially pool effects by state and category
- Uncertainty quantification for “free”
Panel Model for Infant Deaths
- Scales mortality rate to account for variability in state size
Poisson Latent Factor Model
We assume the infant mortality rate in the “no ban” condition can be expressed as
- Model selection problem: choosing
Hierarchical Prior on Causal Effects
Partially pool the exposure parameters
Shrinkage Across States
Shrinkage Across Subcategories
Variation Across Multiple Sources
MCMC Inference
Model implemented in probabilistic programming library, numpyro
MCMC inference with Hamiltonian Monte Carlo
Run multiple chains, check Rhats and effective sample sizes
MCMC Inference
Fit models for each category
- Mortality: Total, race/ethnicity, timing of death and type of death
- Fertility: Total, age, race/ethnicity, education, insurance
For each, fit models for multiple latent ranks and check fit
Code available at:
Model Selection and Checking
- In-sample checks:
- Question: how well does the model fit the observed data
- Tool: gap plots and posterior predictive comparisons
- Used to select latent factor rank
- Out-of-sample checks
- Question: how well can we forecast
- Tool: placebo-in-time checks
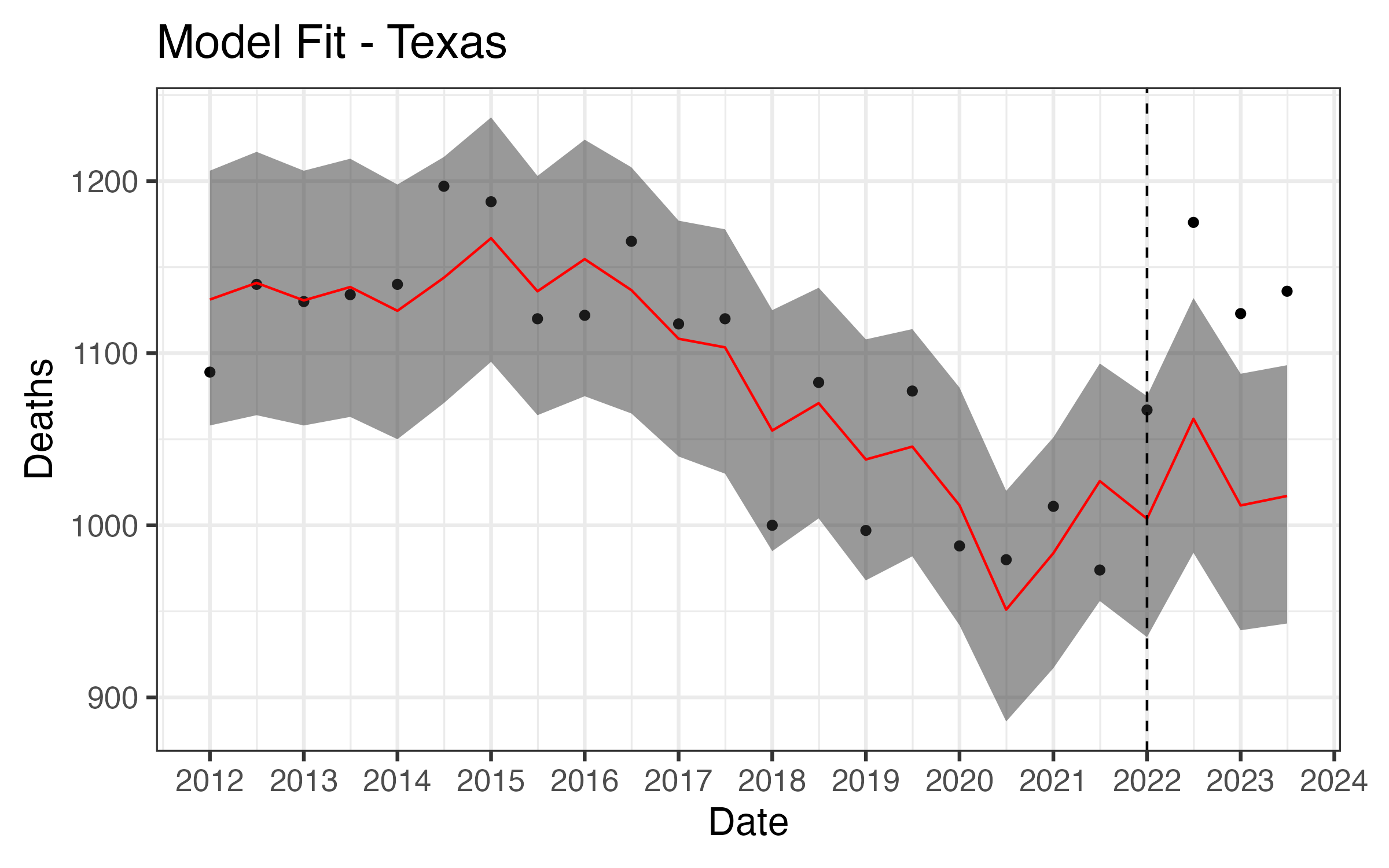
Results - Texas
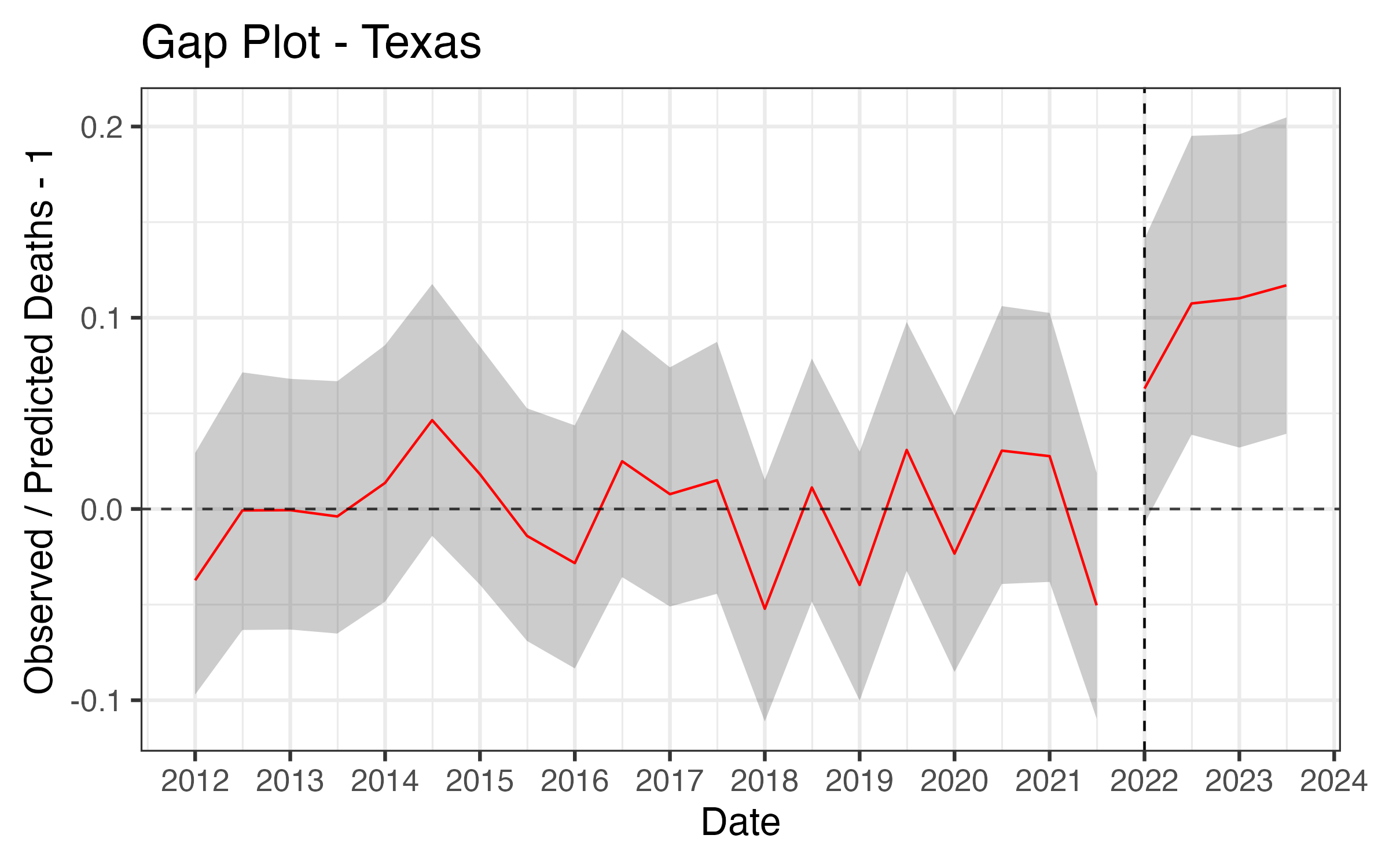
Posterior Predictive Checks
Posterior predictive checks are used to assess how well a Bayesian model fits observed data
Unlike classical hypothesis testing, posterior predictive checks focus on practical significance of model inadequacies
Posterior Predictive Checks
Maximum absolute residual: identify outliers inconsistent with the model:
Residual autocorrelation: check for remaining autocorrelation after controlling latent factors (and seasonal trends)
- Test statistic based on residual autocorrelation at different lags
PPC: Max Residual
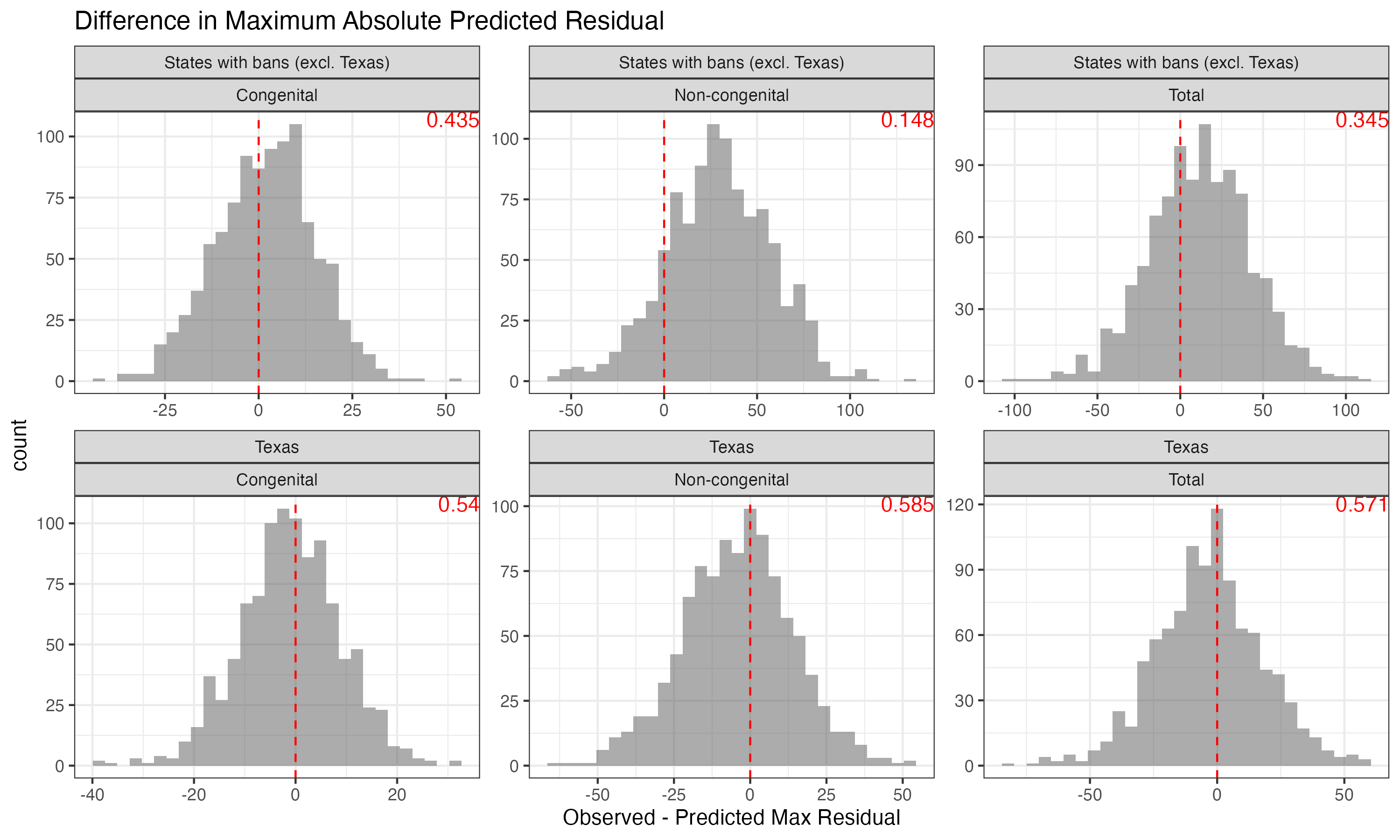
Posterior Predictive Checks
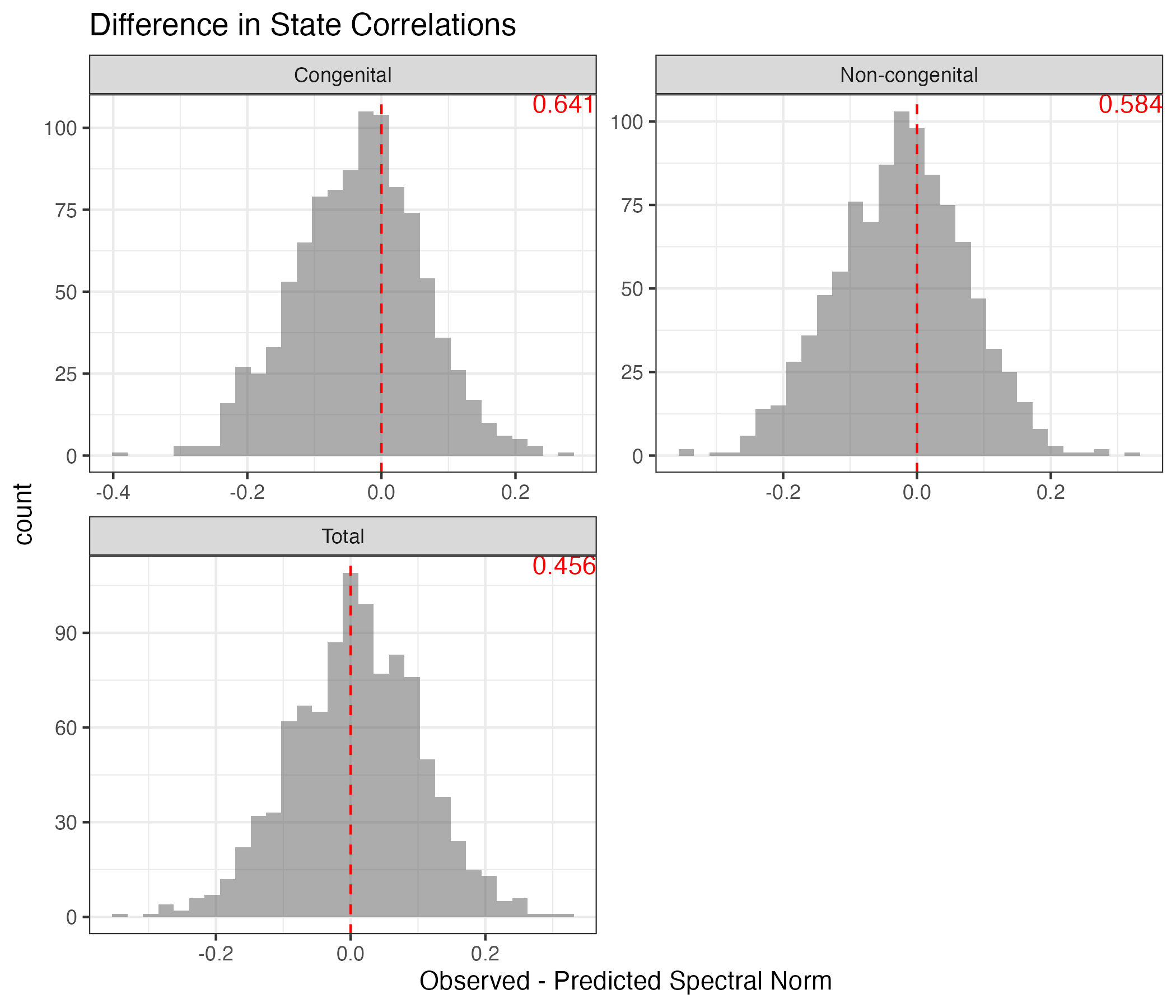
Across-unit correlation: states should be uncorrelated after controlling for latent factors:
- Test statistic based on eigenspectrum of residual correlation matrix
- Let
- T should be small for uncorrelated state-residuals
PPC: State Correlations
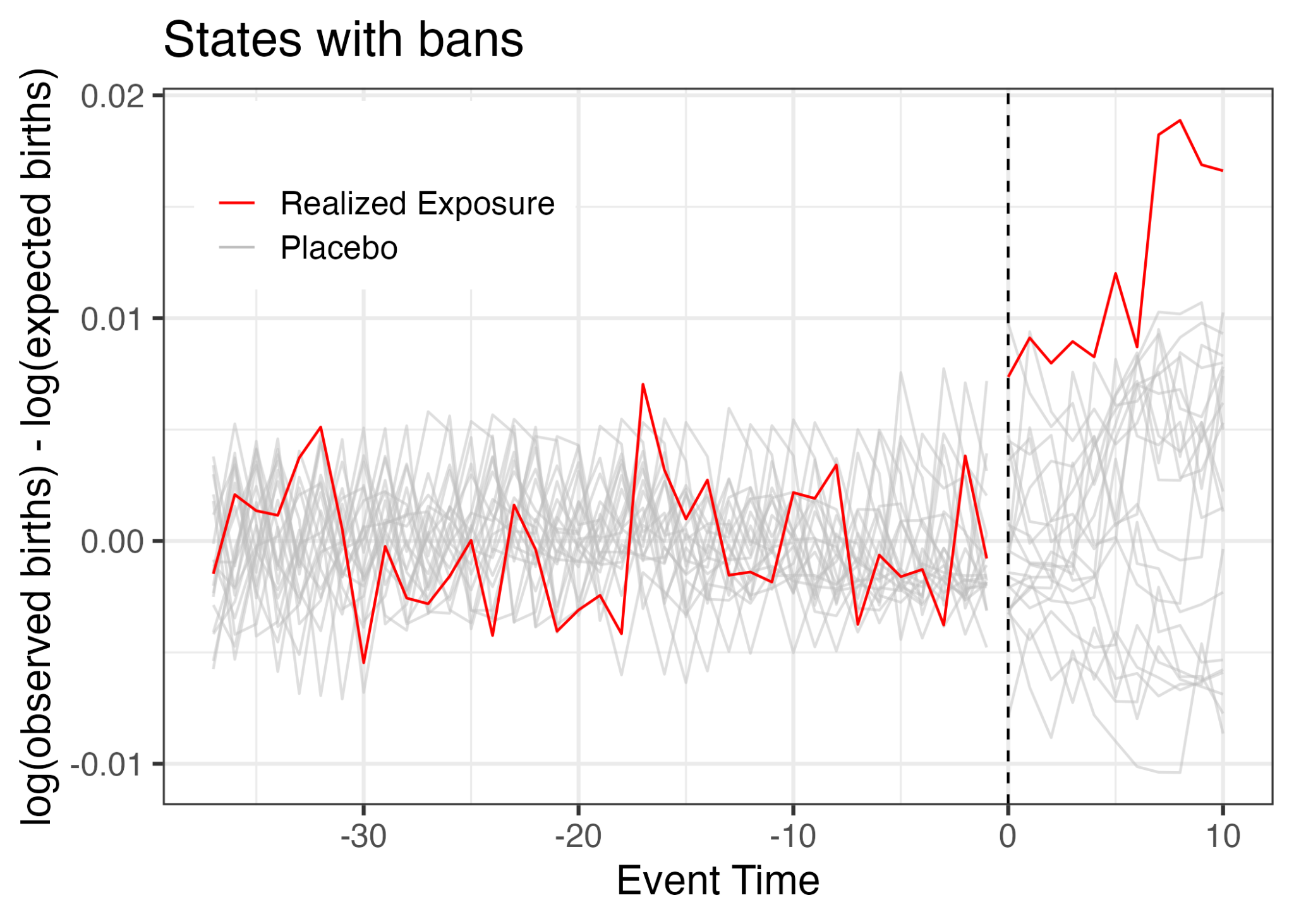
Placebo-in-Time
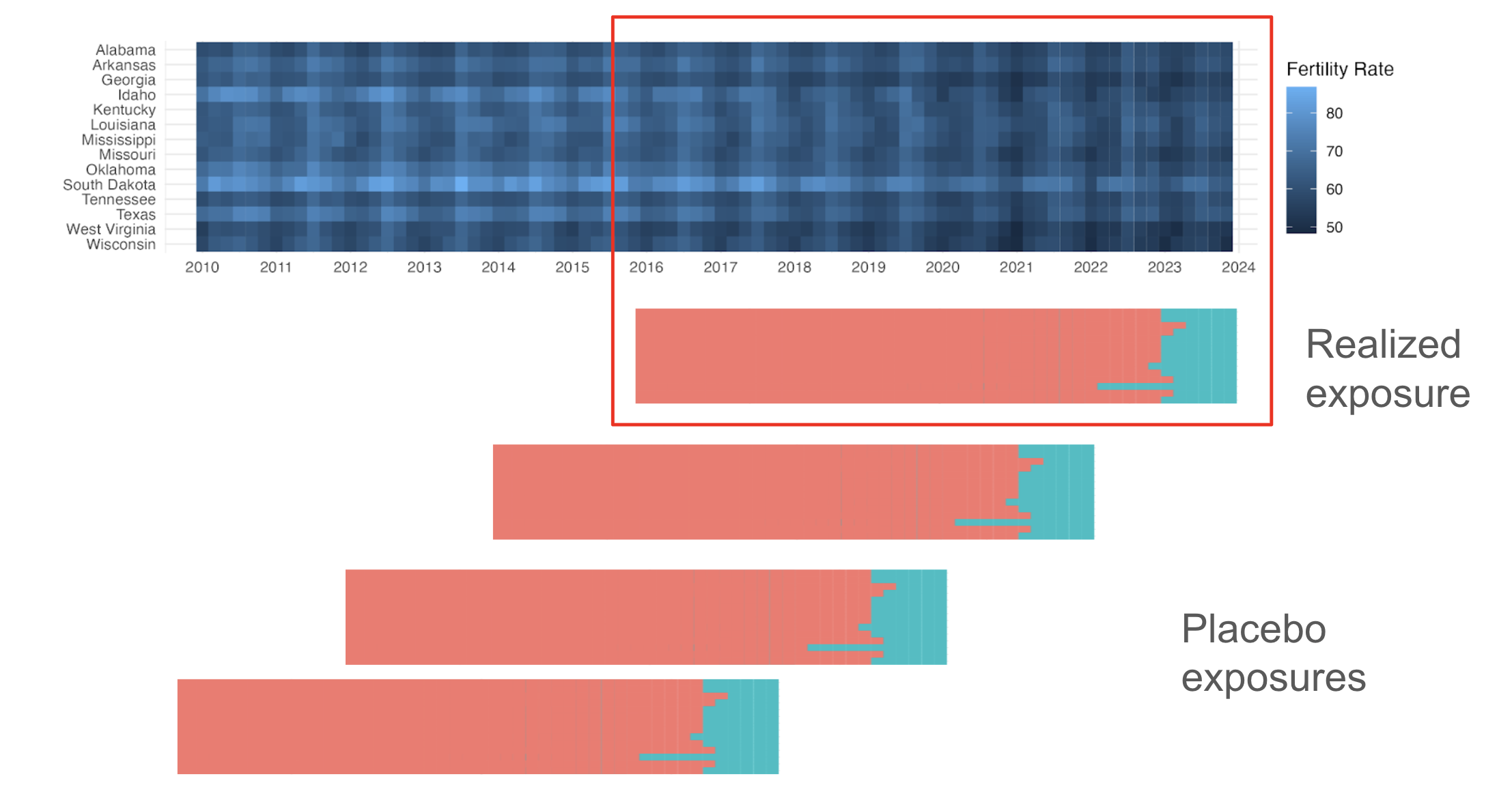
Placebo-in-Time
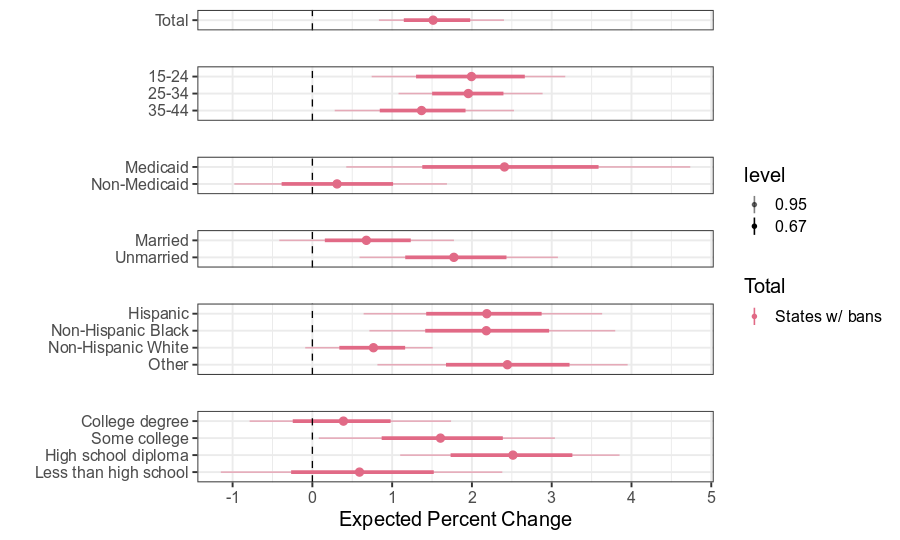
Fertility Impact by Subgroup
State-Specific Effects on Inf. Mortality

In banned states overall, the infant mortality rate increased by 5.6%
- Kentucky: +7.5%
- Texas: +8.9%
Effect on Infant Mortality by Cause
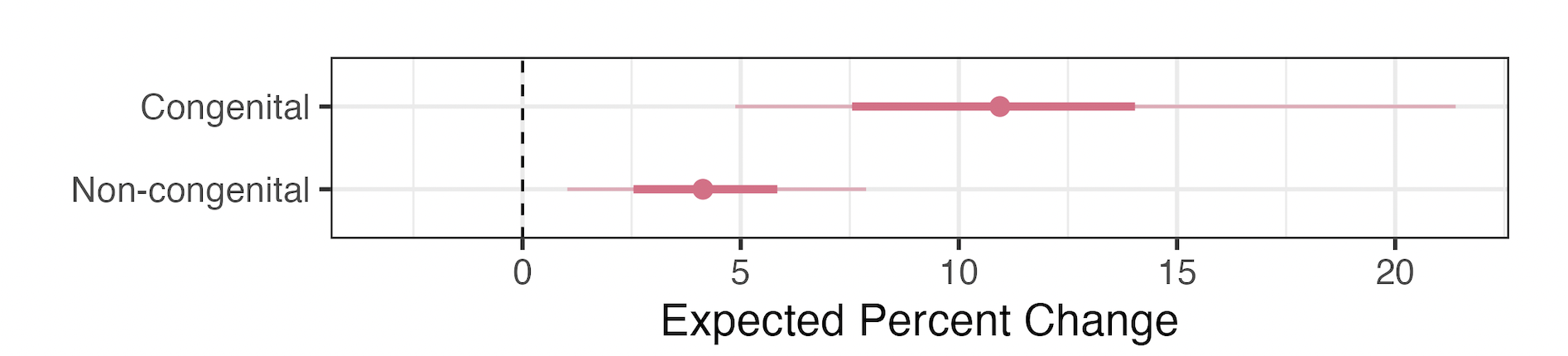
- +10.9% increase in congenital deaths
- +4.2% increase in non-congenital deaths
- Note: majority of deaths attributable to the bans are non-congenital
Effect on Infant Mortality by Race/Ethnicity
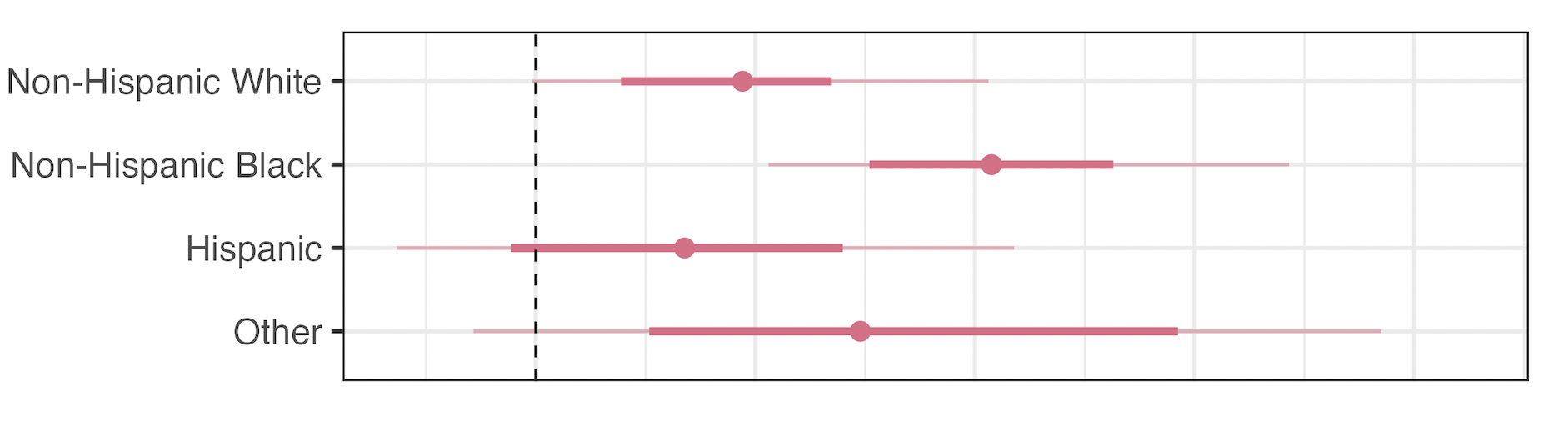
- NH White: +5.1%
- NH Black: +11.0%
- Hispanic: +3.3%
- NH Other: +9.9%
Key Findings
- Strong evidence that birth rates increased above expectation in states that banned abortion (+1.6%)
Slightly smaller than prior studies
Similar in magnitude of recent population-wide events
Largest impacts among those experiencing greatest structural disadvantage (consistent across states)
- Infant mortality increased in states with bans (+5.5%)
- Outsized influence of Texas
- Double the impact among non-Hispanic Black infants
- Larger relative increase among congenital deaths
Implications
- Profound health, social and economic implications of being unable to obtain an abortion (Greene Foster 2020)
- State-specific policies and social contexts may present additional barriers for disadvantaged women
- Bans exacerbate existing health disparities
- Future work: impact of abortion bans on maternal morbidity, high-risk pregnancy care, and birth outcomes (e.g., preterm birth, low birthweight)
Methodological Takeaways
Missing data and staggered adoption are easier to handle with Bayesian models
Hierarchical modeling of the treatment effect in panel data is an underexplored strategy for estimating heterogeneous treatment effects
Choice of temporal aggregation is important and tied to the amount of missingness
More work needed to understand how and when to disaggregate when inferring total effects
Publications


Papers published in JAMA. See Gemmill et al. (2025) and Bell et al. (2025). Supplementary materials contain modeling details.
Thank you!
Additional Slides
Fertility Data
- Bimonthly (e.g., January-February) counts of live births for 50 states and DC from birth certificates for 2014-2023
- Compiled by the National Center for Health Statistics (NCHS)
- 2023 provisional data
- Denominators (women 15-44) by state-year for 2014-2022 (imputed 2023)
- Census: total counts and by age, race/ethnicity
- American Community Survey: proportion by education, marital status, insurance (indirectly)
Fertility Approach
- Outcome: fertility rate (births per 1,000 per year)
- Exposure: 6-week or complete abortion ban (14 states1), staggered adoption
- Pre-policy period: January 2014 through ~December 2022
- Treated period: ~January 2023 through December 2023
- Subgroups
- Age: 15-24, 25-34, 35-44
- Race/ethnicity: non-Hispanic White, non-Hispanic Black, Hispanic, and Other
- Marital status: married, not married
- Educational attainment: <high school, high school degree, some college, college degree+
- Insurance payer for the delivery: Medicaid, non-Medicaid
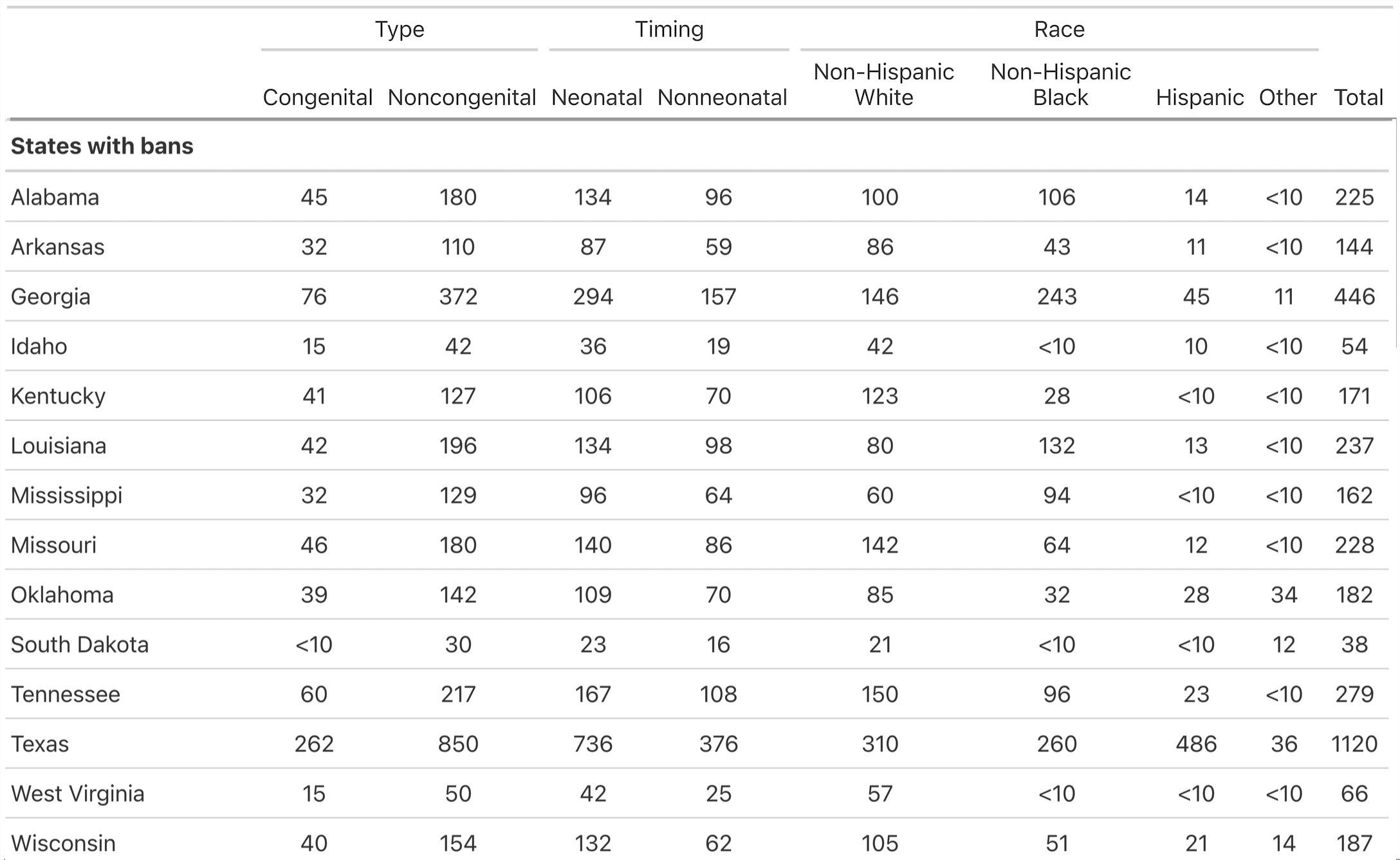
Infant Mortality Data
- Biannual (e.g., January-June) counts of infant deaths (< 1 year) for 50 states and DC from death certificates for 2012-2023
- 2023 provisional data
- Impute suppressed data
- Denominators (live births) by state-biannual period for 2012-2023 from birth certificates
Missing Data
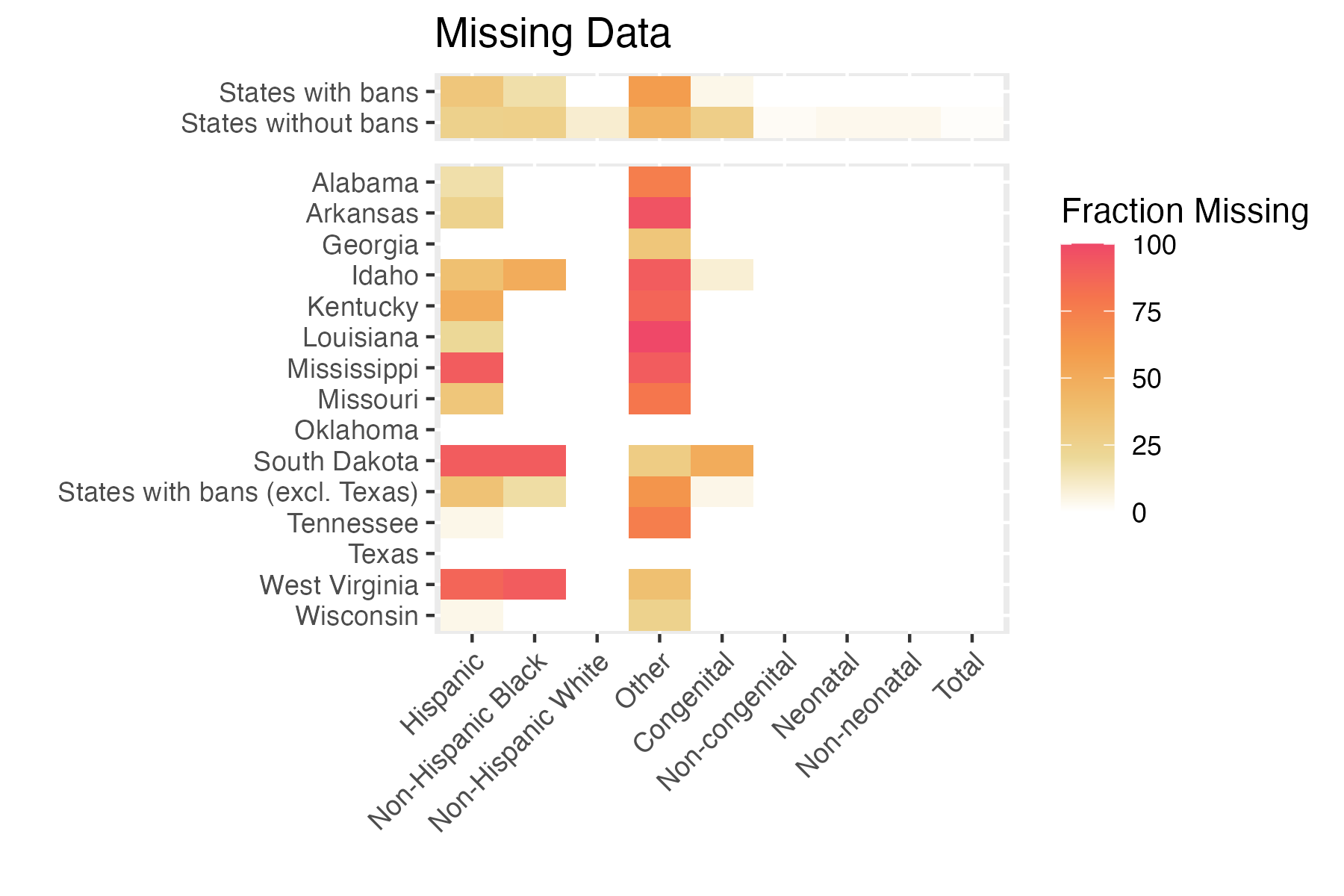
Note: missingness depends on level of temporal aggregation
Median Infant Deaths per Half-Year
State-Specific Effects on Fertility Rate
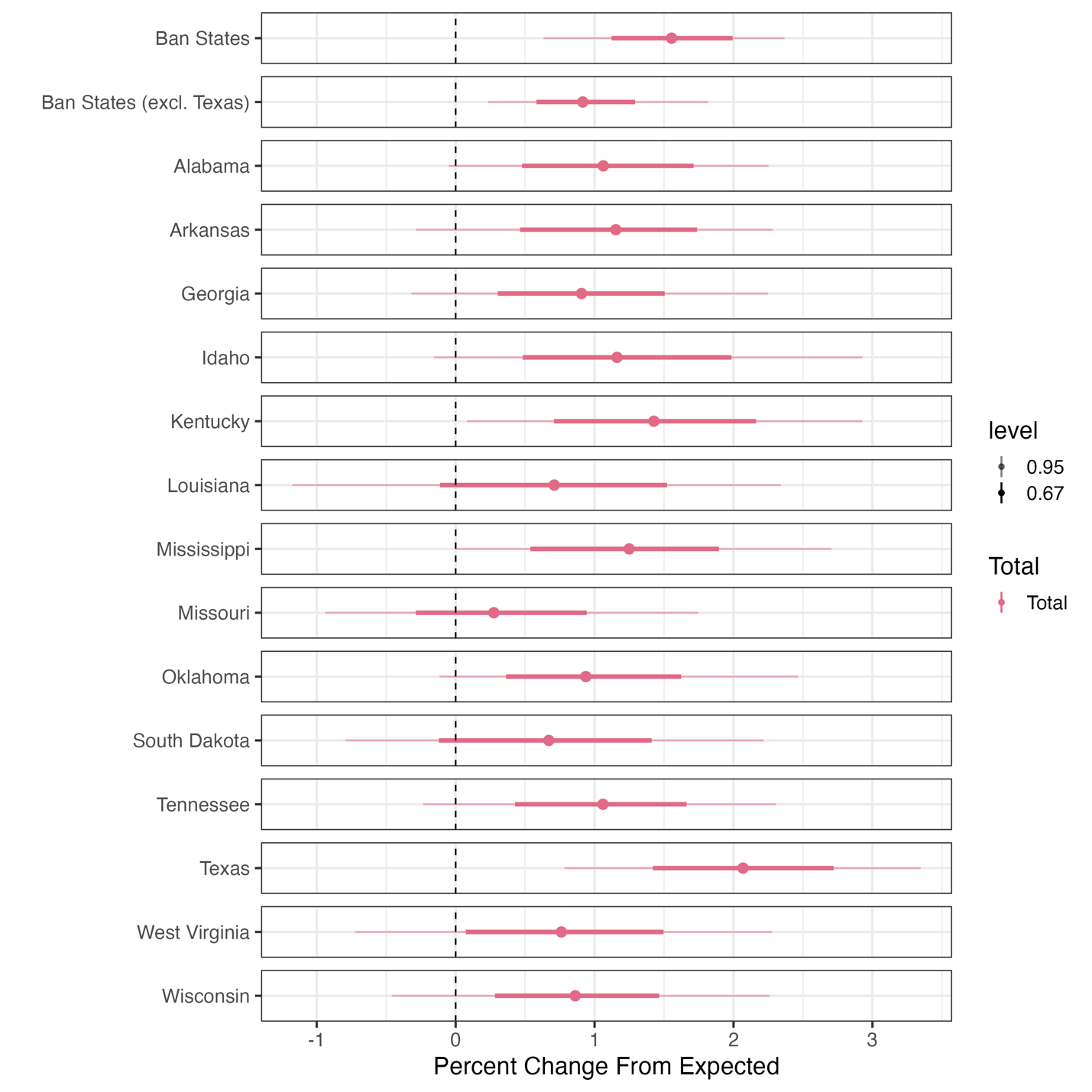
Range: 0.6% - 2.1%
Overall: +1.7%
Non-Texas: +0.9%
Likelihood - Infant Mortality
Let
RAND - Stat Group Seminar